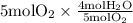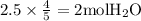Chemistry, 04.07.2019 21:50, Jasmine3864
The combustion of propane (c3h8) produces co2 and h2o: c3h8 (g) + 5o2 (g) → 3co2 (g) + 4h2o (g) the reaction of 2.5 mol of o2 with 4.6 mol of c3h8 will produce mol of h2o.

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
Do you know the correct answer?
The combustion of propane (c3h8) produces co2 and h2o: c3h8 (g) + 5o2 (g) → 3co2 (g) + 4h2o (g) the...
Questions in other subjects:


Physics, 10.11.2019 11:31




Mathematics, 10.11.2019 11:31

Advanced Placement (AP), 10.11.2019 11:31

Mathematics, 10.11.2019 11:31

History, 10.11.2019 11:31


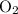 with 4.6 mol
with 4.6 mol 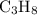 will produce 2mol
will produce 2mol 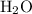 .
.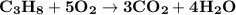
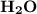 produced will be,
produced will be,