
Chemistry, 04.07.2019 07:50, jacobmclawhorn3001
Covalent solutes are considered non-electrolytes. what does this mean for the conductivity of the solution? a) non-electrolytes dissolve and completely dissociate in water providing charged ions to conduct electricity. b) non-electrolytes dissolve and do not dissociate in water providing no charged ions to conduct electricity. c) being a non-electrolyte refers the type of bonding and has no bearing on conducting electricity. d) non-electrolytes do not dissolve in water and can not conduct electricity.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 06:30, backup5485
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1


Chemistry, 22.06.2019 17:00, calmicaela12s
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
Do you know the correct answer?
Covalent solutes are considered non-electrolytes. what does this mean for the conductivity of the so...
Questions in other subjects:



English, 19.11.2020 14:00




Mathematics, 19.11.2020 14:00

English, 19.11.2020 14:00

English, 19.11.2020 14:00


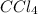 in water will act as a non-electrolyte.
in water will act as a non-electrolyte.




