
Chemistry, 03.07.2019 08:00, mariahisawsome1
An is a hydrocarbon in which one or more hydrogen atoms is replaced by a -coh group. a. aldehyde b. alcohol c. ester d. amino acid

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, sierravick123owr441
Asap! ! who wants to me with my assignment : ) (especially with how i’m gonna draw out the example) you have explored some interesting, informative, and amusing examples of models. now it's time to get creative and make your own model. here is the requirement checklist for your model: ✔ model types can include drawings, diagrams, physical models, virtual simulations, or videos. ✔ models must be created by you, not something selected from an online or outside source. ✔ submit a presentation, picture, video, or screenshot of your model. ✔ submit a one-paragraph summary describing the topic you chose, your model, what it represents, how you made it, and the specific science involved. it is important that you are using science terminology and are accurate. now that you know how to create and submit your model, you will need to choose a topic for your model. choose one of the three topics listed below. select each topic for an overview. -conservation of mass -atomic theory -thermal energy
Answers: 2



Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1
Do you know the correct answer?
An is a hydrocarbon in which one or more hydrogen atoms is replaced by a -coh group. a. aldehyde b....
Questions in other subjects:

Health, 27.02.2020 05:39







English, 27.02.2020 05:39

Chemistry, 27.02.2020 05:39


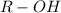 .
.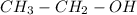 is obtained when one hydrogen atom is replaced by
is obtained when one hydrogen atom is replaced by 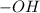 group.
group.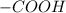 and ammine group,
and ammine group, 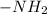 .
.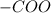 group is attached to a hydrocarbon is known as ester.
group is attached to a hydrocarbon is known as ester.
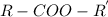 . It is directly attached to the two alkyl group of carbon.
. It is directly attached to the two alkyl group of carbon. .
.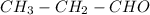 is obtained when one hydrogen atom is replaced by
is obtained when one hydrogen atom is replaced by 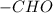 group.
group.



