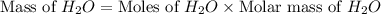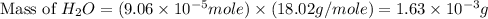Chemistry, 03.07.2019 05:00, rose782751
2h2 +o2 → 2h2o what mass of water forms when 1.45 × 10-3 g o2 react completely? (molar mass of o2 = 32.00 g/mol; molar mass of h2o = 18.02 g/mol) 1.63 × 10-3 g 8.16 × 10-4 g 1.29 × 10-3 g

Answers: 1
Other questions on the subject: Chemistry



Do you know the correct answer?
2h2 +o2 → 2h2o what mass of water forms when 1.45 × 10-3 g o2 react completely? (molar mass of o2 =...
Questions in other subjects:

Mathematics, 24.11.2020 16:30


Computers and Technology, 24.11.2020 16:30

Mathematics, 24.11.2020 16:30


Mathematics, 24.11.2020 16:30

History, 24.11.2020 16:30

History, 24.11.2020 16:30

Mathematics, 24.11.2020 16:30


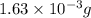
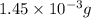
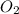 .
.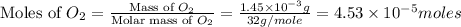
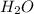 .
.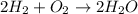
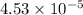 moles of
moles of 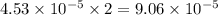 moles of
moles of