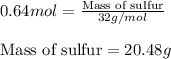Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, litttyyyu33411
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2


Chemistry, 22.06.2019 14:00, coylenoah0
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
Do you know the correct answer?
Sulfur and fluorine react in a combination reaction to produce sulfur hexafluoride: s(g) + 3f2(g) -...
Questions in other subjects:

Mathematics, 30.04.2021 16:10

Social Studies, 30.04.2021 16:10



Mathematics, 30.04.2021 16:10





History, 30.04.2021 16:10


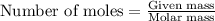 .....(1)
.....(1)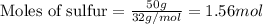
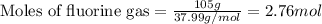
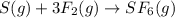
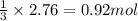 of sulfur
of sulfur