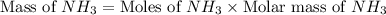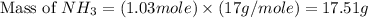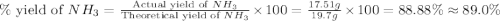Chemistry, 12.10.2019 14:30, jademckinziemea
Nitrogen gas (n2) reacts with excess hydrogen gas (h2) to produce ammonia (nh3). what is the percent yield of ammonia if the actual yield is 1.03 moles and the theoretical yield is 19.7 grams?
a. 17.5%
b. 35.0%
c. 52.3%
d. 89.0%

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:30, nuclearfire278
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease. correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 16:40, roderickhinton
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
Do you know the correct answer?
Nitrogen gas (n2) reacts with excess hydrogen gas (h2) to produce ammonia (nh3). what is the percent...
Questions in other subjects:

Chemistry, 24.04.2020 04:56

Social Studies, 24.04.2020 04:56



English, 24.04.2020 04:56

History, 24.04.2020 04:56


Mathematics, 24.04.2020 04:57

Mathematics, 24.04.2020 04:57

Mathematics, 24.04.2020 04:57


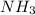 = 1.03 mole
= 1.03 mole