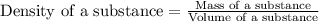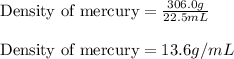Chemistry, 02.07.2019 13:10, oddoneshenchman
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml. the mercury used to fill the cylinder weighs 306.0 g. from this information, calculate the density of mercury.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1



Chemistry, 23.06.2019 13:30, MalikaJones
Determine the rate law, including the values of the orders and rate law constant, for the following reaction using the experimental data provided. a + b yields products trial [a] [b] rate 1 0.30 m 0.25 m 1.2 × 10-2 m/min 2 0.30 m 0.50 m 4.8 × 10-2 m/min 3 0.60 m 0.50 m 9.6 × 10-2 m/min
Answers: 1
Do you know the correct answer?
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml. the mercury used to fi...
Questions in other subjects:

Physics, 03.08.2019 07:30


Mathematics, 03.08.2019 07:30




Mathematics, 03.08.2019 07:30

Chemistry, 03.08.2019 07:30


Mathematics, 03.08.2019 07:30