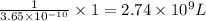Chemistry, 02.07.2019 07:00, familyvazquez7
At 25.0∘c, the molar solubility of silver chromate in water is 1.10×10−12 m . calculate the solubility in grams per liter. express your answer in grams per liter to three significant figures. how many liters of water are required to dissolve 1.00 g of silver chromate? express your answer in liters to three significant figures.

Answers: 2
Other questions on the subject: Chemistry



Chemistry, 23.06.2019 06:40, Taylor73836
4786 joules of heat are transferred to a 89.0 gramsample of an unknown material, with an initialtemperature of 23.0°c. what is the specific heat of thematerialif the final temperature is 89.5 °c?
Answers: 1

Chemistry, 23.06.2019 07:00, jstyopin
In order for a high temperature boiler or steam engine to produce superheated water, or steam: the heat source must be greater than 100°c the water must be permitted to evaporate quickly the system must be sealed and become pressurized above atmospheric pressure the vapor pressure must be kept below 760 mm(hg)
Answers: 1
Do you know the correct answer?
At 25.0∘c, the molar solubility of silver chromate in water is 1.10×10−12 m . calculate the solubili...
Questions in other subjects:

Mathematics, 21.01.2020 11:31




Computers and Technology, 21.01.2020 11:31




Social Studies, 21.01.2020 11:31

History, 21.01.2020 11:31


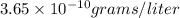 and
and 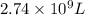 of water is required to dissolve 1 g of silver chromate.
of water is required to dissolve 1 g of silver chromate.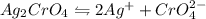
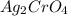 gives 2 moles of
gives 2 moles of 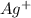 and 1 mole of
and 1 mole of 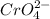
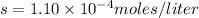
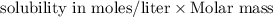
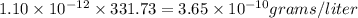
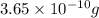 of
of