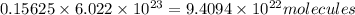Chemistry, 01.07.2019 07:20, dtrdtrdtrdtrdrt1325
An open flask sitting in a lab fridge looks empty, but we know that actually it is filled with a mixture of gases called air. if the flask volume is 3.50 l, and the air is at standard temperature and pressure, how many gaseous molecules does the flask contain?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, babygirlqueen5588
How many electrons does silver have to give up in order to achieve a sido noble gas electron configuration?
Answers: 3

Chemistry, 22.06.2019 20:20, Matseleng3775
The characteristics of two different types of reactions are shown below: reaction a: electrons are gained by the atoms of an element. reaction b: protons are lost by the atom of an element. which statement is true about the atoms of the elements that participate in the two reactions? their identity changes in both reaction a and reaction b. their identity changes in reaction a but not in reaction b. their identity changes in reaction b but not in reaction a. their identity remains the same in both reaction a and reaction b.
Answers: 1

Chemistry, 23.06.2019 01:10, dontcareanyonemo
Can someone check my work 98 5.05 acids and bases for this assignment you will be comparing acids and bases. the chart below will you organize the information needed: acids bases chemical properties (2) deodorant detergent vinger dish soap physical properties (2) orange juice toilet cleaner drain cleaner window cleaner ph level acid ph goes from 0-4 bases ph goes from 10-14 examples around you (2) vinger coffee lemon juice dark chocolate
Answers: 3

Chemistry, 23.06.2019 11:40, brendonvernon8
Which of the following would have the lowest average kinetic energy
Answers: 1
Do you know the correct answer?
An open flask sitting in a lab fridge looks empty, but we know that actually it is filled with a mix...
Questions in other subjects:

Mathematics, 20.10.2020 22:01

Chemistry, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Arts, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01



Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01

Mathematics, 20.10.2020 22:01


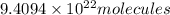 of air.
of air.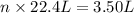
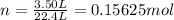
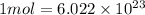 atoms/molecules
atoms/molecules