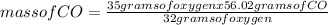When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many grams of co can be formed from 35.0 grams of oxygen? 2c + o2 → 2co using 32.00 g/mole as the molecular mass of oxygen and 28.01 g/mole as the molecular mass of carbon monoxide, solve the above problem.

Answers: 1
Similar questions

Chemistry, 05.10.2019 09:30, sosick3595
Answers: 1


Chemistry, 16.10.2019 18:00, willveloz4
Answers: 1

Chemistry, 31.10.2019 20:31, genesis6154
Answers: 1
Do you know the correct answer?
When the amount of oxygen is limited, carbon and oxygen react to form carbon monoxide. how many gram...
Questions in other subjects:


Physics, 30.11.2019 12:31


Mathematics, 30.11.2019 12:31


Social Studies, 30.11.2019 12:31

Mathematics, 30.11.2019 12:31

Biology, 30.11.2019 12:31

English, 30.11.2019 12:31

Mathematics, 30.11.2019 12:31


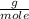 O₂: 32
O₂: 32