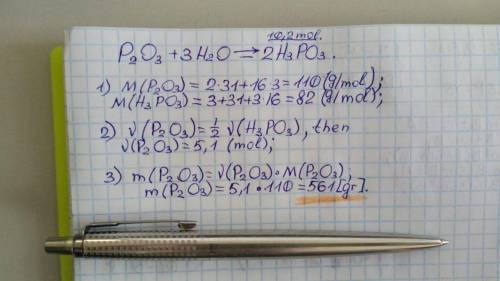Chemistry, 18.04.2022 04:40, yansaturnin
Consider the balanced reaction
below:
P2O3 + 3H2O → 2H3PO3
How many grams of diphosphorus
trioxide, P203, are required to
produce 10.2 moles of phosphorous
acid, H3PO3?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 05:30, stellaglenn205
What reaction is taking place? 02 + c3h8 = h20 + co2
Answers: 1

Do you know the correct answer?
Consider the balanced reaction
below:
P2O3 + 3H2O → 2H3PO3
How many grams of diphospho...
P2O3 + 3H2O → 2H3PO3
How many grams of diphospho...
Questions in other subjects:

Chemistry, 02.02.2021 01:20



Computers and Technology, 02.02.2021 01:20

Mathematics, 02.02.2021 01:20




Mathematics, 02.02.2021 01:20