Carbon disulfide is produced by the reaction listed below:
--->
If you started the...


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, chefdnguyen
How many liters of water vapor can be produced if 108 grams of methane gas (ch4) are combusted at 312 k and 0.98 atm? show all work. pls ! will mark as brainliest
Answers: 1

Chemistry, 22.06.2019 05:30, tifftiff22
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2

Do you know the correct answer?
Questions in other subjects:




Chemistry, 05.02.2020 00:49


Mathematics, 05.02.2020 00:49


Health, 05.02.2020 00:49



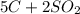 --->
--->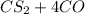
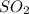 and excess carbon, what amount, in moles, of
and excess carbon, what amount, in moles, of 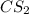 will be produced?
will be produced?




