
Chemistry, 24.03.2022 14:00, lollipop83
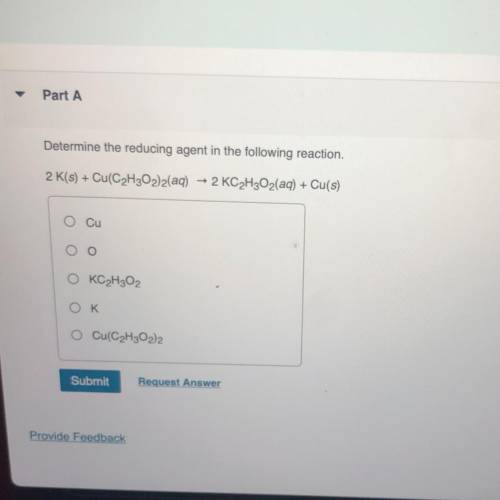
Determine the reducing agent in the following reaction. 2 K(s) + Cu(C2H302)2(aq) + 2 KC2H2O2(aq) + Cu(s)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:20, andybiersack154
Asolution is made by dissolving 25.5 grams of glucose (c6h12o6) in 398 grams of water. what is the freezing point depression of the solvent if the freezing point constant is -1.86 °c/m? show all of the work needed to solve this problem.
Answers: 1


Chemistry, 22.06.2019 09:00, heids17043
Chen drew a diagram to compare the ways in which different organisms obtain nitrogen. which label belongs to the area marked z?
Answers: 3
Do you know the correct answer?
Determine the reducing agent in the following reaction.
2 K(s) + Cu(C2H302)2(aq) + 2 KC2H2O2(aq) +...
Questions in other subjects:





English, 15.01.2021 23:40





Biology, 15.01.2021 23:40






