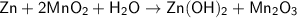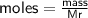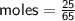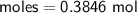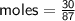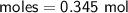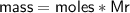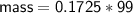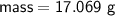Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, Sbeech7246
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2


Chemistry, 22.06.2019 19:30, 2020sanchezyiczela
Draw the lewis structure for the trisulfur s3 molecule. be sure to include all resonance structures that satisfy the octet rule.
Answers: 3

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
Do you know the correct answer?
Zn + 2MnO2 + H2O → Zn(OH)2 + Mn2O3
Determine the limiting reactant if 25g of Zn and 30g of MnO2 ar...
Questions in other subjects:


Mathematics, 14.12.2020 23:30





English, 14.12.2020 23:30


Mathematics, 14.12.2020 23:30

Arts, 14.12.2020 23:30