Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 23.06.2019 03:00, draveon6925
Achemical equilibrium between gaseous reactants and products is shown. n2(g) + 3h2(g) ⇌ 2nh3(g) how will the reaction be affected if the pressure on the system is increased? it will shift toward the reactant side as there is lower pressure on the reactant side. it will shift toward the product side as there is higher pressure on the product side. it will shift toward the reactant side as there are a greater number of moles of gas on the reactant side. it will shift toward the product side as there are a fewer number of moles of gas on the product side.
Answers: 2
Do you know the correct answer?
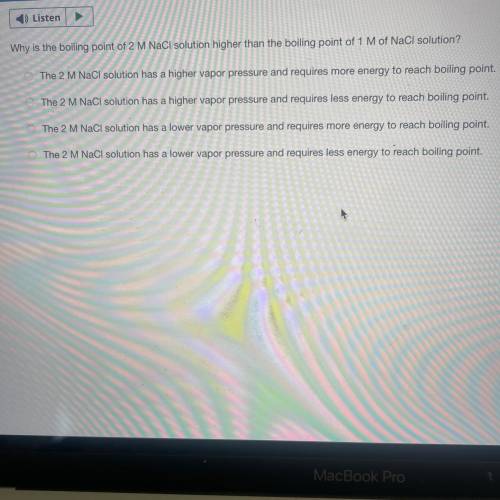
Why is the boiling point of 2 M NaCl solution higher than the boiling point of 1 M of NaCI solution?...
Questions in other subjects:

History, 02.12.2020 07:20

Mathematics, 02.12.2020 07:20

Mathematics, 02.12.2020 07:20


History, 02.12.2020 07:20

Mathematics, 02.12.2020 07:20

History, 02.12.2020 07:20


History, 02.12.2020 07:20

History, 02.12.2020 07:20