
Chemistry, 12.03.2022 18:30, tahjaybenloss16
the slowest step in a reaction mechanism requires the collision represented above to occur. Which of the following most likely indicates how the addition of a solid catalyst could increase the rate of the reaction?
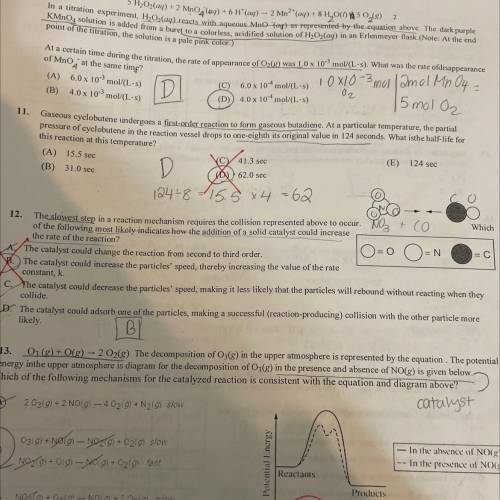

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, mandy9386
You are making a solution of calcium chloride dissolved in water. you add solid, stir, and it dissolves. you add just a spatula tip full, stir, and the solid does not dissolve. how could you describe the solutions before and after adding the spatula tip amount
Answers: 1

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 23.06.2019 05:50, kawaunmartinjr10
Aseismic wave is energy released as the result of rock movement along a fault. t or f ?
Answers: 1

Chemistry, 23.06.2019 09:20, taylorannsalazar
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
Do you know the correct answer?
the slowest step in a reaction mechanism requires the collision represented above to occur. Which of...
Questions in other subjects:



Physics, 12.08.2020 05:01

Biology, 12.08.2020 05:01

History, 12.08.2020 05:01

Physics, 12.08.2020 05:01

Mathematics, 12.08.2020 05:01



Mathematics, 12.08.2020 05:01






