
Chemistry, 06.03.2022 21:50, tressasill
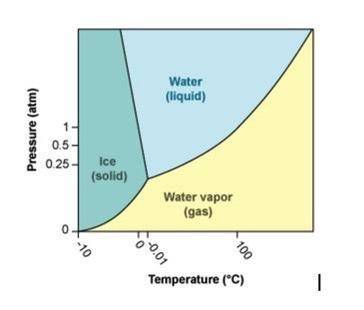
Use the phase diagram for H2O to answer the following questions.
a. What phase is water in at 100°C and 2 atm pressure? (1 point)
b. What happens to water at 100°C as pressure is increased from 0.8 atm to 1.2 atm? (1 point)
c. What happens to water at 1 atm pressure as the temperature is decreased from 10°C to –10°C? (1 point)
d. What line represents boiling points on a phase diagram? (You may describe it or label it on the phase diagram.) (1 point)
e. How do intermolecular forces and kinetic energy interact to determine at what point a liquid will boil? (2 points)

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 22.06.2019 22:00, choatefarmsus
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 10:30, yaaaaa31gghgf
Ethyl alcohol, also known as ethanol, has a density of 0.79 g/ml. what is the volume, in quarts, of 1.95 kg of this alcohol?
Answers: 2
Do you know the correct answer?
Use the phase diagram for H2O to answer the following questions.
a. What phase is water in at 100°...
Questions in other subjects:





Biology, 24.06.2021 01:00



Mathematics, 24.06.2021 01:00


Mathematics, 24.06.2021 01:00






