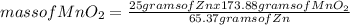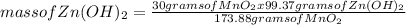Chemistry, 26.02.2022 08:50, sebastianmettsovghhk
What mass of zinc hydroxide (Zn(OH)2) will be produced if 25.0g Zn and 30.0g MnO2 react in a battery according to the following reaction: Be sure to check the limiting reactant. Zn + 2MnO2 + H2O → Zn(OH)2 + Mn2O3

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, bossboybaker
Select the correct answer. which phrase correctly describes temperature? o a. average rotational kinetic energy of the particles in an object o b. average energy of the particles in an object c. average translational kinetic energy of the particles in an object od. all energy possessed by the particles in an object
Answers: 1

Chemistry, 22.06.2019 14:00, emilyproce
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1


Chemistry, 22.06.2019 16:50, briansalazar17
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
Do you know the correct answer?
What mass of zinc hydroxide (Zn(OH)2) will be produced if 25.0g Zn and 30.0g MnO2 react in a battery...
Questions in other subjects:

English, 15.11.2020 04:10

History, 15.11.2020 04:10






Mathematics, 15.11.2020 04:10

Arts, 15.11.2020 04:10