
Chemistry, 25.02.2022 07:10, Marshmallow6989
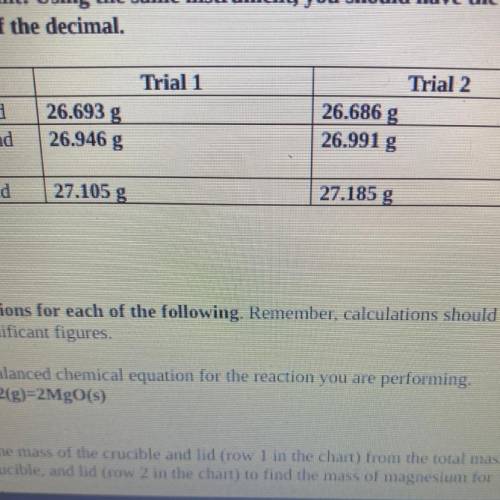
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical
yield of Mgo for each trial.
. Trial 1:
• Trial 2:
5. Determine the percent yield of Mgo for your experiment for each trial.
Trial 1:
• Trial 2:
6. Determine the average percent yield of Mgo for the two trials.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 21.06.2019 19:40, raquelqueengucci25
In this synthesis reaction what products will form
Answers: 1

Chemistry, 22.06.2019 03:30, fbillinton
In this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced?
Answers: 1

Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
Do you know the correct answer?
4. Magnesium is the limiting reactant in this experiment. Calculate the theoretical
yield of Mgo f...
Questions in other subjects:


Mathematics, 07.01.2021 05:50

Biology, 07.01.2021 05:50


History, 07.01.2021 05:50

Social Studies, 07.01.2021 05:50


Physics, 07.01.2021 05:50







