1.Which of these statements about the
conservation of mass is not correct?
A. The mass of th...

Chemistry, 24.02.2022 09:10, stalley1521
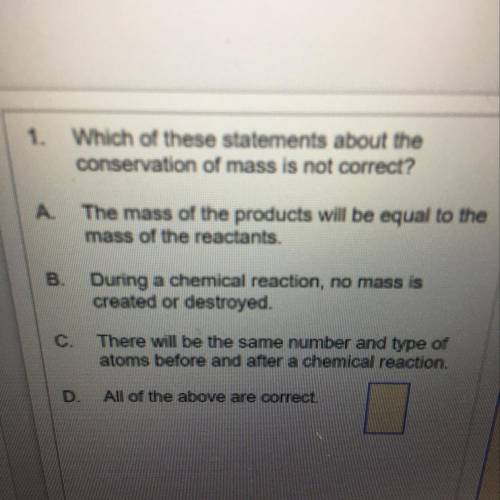
1.Which of these statements about the
conservation of mass is not correct?
A. The mass of the products will be equal to the
mass of the reactants.
B. During a chemical reaction, no mass is
created or destroyed.
C. There will be the same number and type of
atoms before and after a chemical reaction.
D. All of the above are correct.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 06:10, gabriellestaleyga16
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 12:00, luffybunny
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 12:00, carvajalj2520
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Geography, 20.01.2020 04:31


History, 20.01.2020 04:31

Physics, 20.01.2020 04:31




Business, 20.01.2020 04:31






