
Chemistry, 24.02.2022 01:00, samantha636
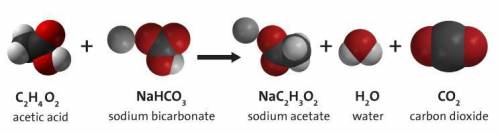
Carbon dioxide gas can be prepared by the reaction of acetic acid and sodium
If 94.46g of sodium bicarbonate are completely reacted, how many liters of carbon dioxide are produced?
Remember to use 1 decimal place when calculating molar mass.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:50, lejeanjamespete1
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1


Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1

Chemistry, 23.06.2019 01:00, ZaNiyahlove4711
Which of the following is the molecular formula for a simple sugar? a. cooh b. h2o c. oh d. c6h12o6
Answers: 1
Do you know the correct answer?
Carbon dioxide gas can be prepared by the reaction of acetic acid and sodium
If 94.46g of sodium b...
Questions in other subjects:

History, 29.06.2019 14:20



History, 29.06.2019 14:20

Arts, 29.06.2019 14:20

Arts, 29.06.2019 14:20

Social Studies, 29.06.2019 14:20

World Languages, 29.06.2019 14:20


Chemistry, 29.06.2019 14:20






