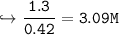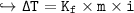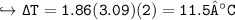5 points
15. Given the formula below: What is the change in the water's freezing
point when...

Chemistry, 22.02.2022 03:20, keilahsalmon
5 points
15. Given the formula below: What is the change in the water's freezing
point when 75 grams of NaCl is added to 400 grams of H20? (Kf water -
1.86 C/m) *
Change in Temp = - ix K, x m
- 0.119 ° C
- 1.19 ° C
O A
B.
- 11.9°C
- 119°C
C

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, asanchez4292
What type of matter is made of only one kind of atom
Answers: 2

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
Do you know the correct answer?
Questions in other subjects:


Mathematics, 18.09.2019 20:00

English, 18.09.2019 20:00

Mathematics, 18.09.2019 20:00


Biology, 18.09.2019 20:00