
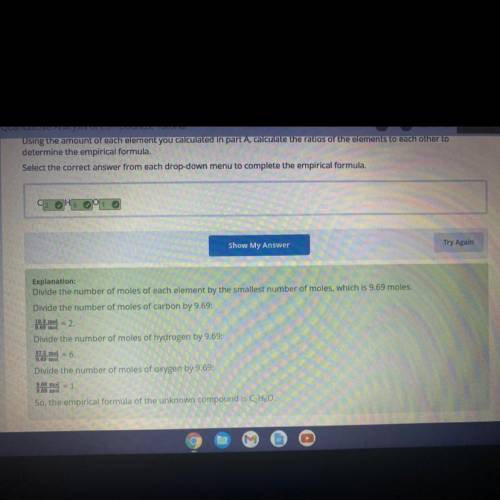
Using the amount of each element you calculated in part A, calculate the ratios of the elements to each other to
determine the empirical formula.
Select the correct answer from each drop-down menu to complete the empirical formula.
CHO
(Answer for those who need help)

Answers: 1
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 12:00, Alexislol7908
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 17:30, tiffanyhmptn
Which scenario is most similar to the type of collision that gas particles have according to kinetic molecular theory
Answers: 1
Do you know the correct answer?
Using the amount of each element you calculated in part A, calculate the ratios of the elements to e...
Questions in other subjects:

English, 13.01.2021 17:10






Physics, 13.01.2021 17:10


Chemistry, 13.01.2021 17:10








