
Chemistry, 14.02.2022 09:00, whereswoodruff
The following molecular equation represents the reaction that occurs when aqueous solutions of silver(I) nitrate and barium chloride are combined.
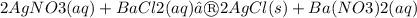
Write the balanced net ionic equation for the reaction.
(Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.)

Answers: 3
Other questions on the subject: Chemistry



Do you know the correct answer?
The following molecular equation represents the reaction that occurs when aqueous solutions of silve...
Questions in other subjects:

English, 16.10.2020 23:01

Chemistry, 16.10.2020 23:01

Physics, 16.10.2020 23:01


History, 16.10.2020 23:01

Chemistry, 16.10.2020 23:01

History, 16.10.2020 23:01

History, 16.10.2020 23:01

Mathematics, 16.10.2020 23:01

Chemistry, 16.10.2020 23:01







