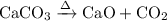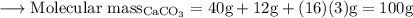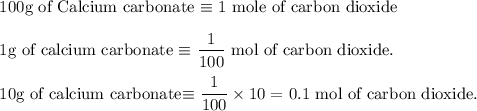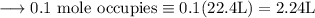Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1


Chemistry, 23.06.2019 07:30, bryantjorell
Which statement is actually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 2
Do you know the correct answer?
Write an equation for the thermal decomposition of CaCO3. Determine the volume of CO2 measured at s...
Questions in other subjects:




Computers and Technology, 27.09.2019 23:10






Computers and Technology, 27.09.2019 23:10