
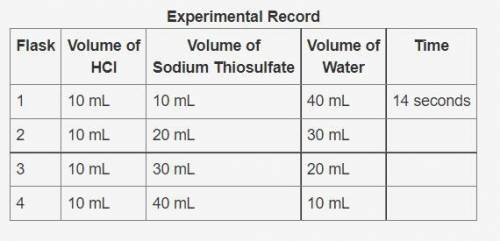
In an experiment, hydrochloric acid reacted with different volumes of sodium thiosulfate in water. A yellow precipitate was formed during the reaction. A cross drawn at the base of each flask became gradually invisible due the formation of this yellow precipitate. The time taken for the cross to become invisible was recorded. A partial record of the experiment is shown.
Experimental Record
Flask Volume of
HCl Volume of
Sodium Thiosulfate Volume of
Water Time
1 10 mL 10 mL 40 mL 14 seconds
2 10 mL 20 mL 30 mL
3 10 mL 30 mL 20 mL
4 10 mL 40 mL 10 mL
Based on your knowledge of factors that affect the rates of chemical reactions, predict the trend in the last column of the experimental record. Use complete sentences to explain the trend you predicted. You do not have to determine exact values for time; just describe the trend you would expect (increase or decrease) and why it occurs.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 23.06.2019 15:30, kfull6027
The amount of iron in ore can be quantitatively determined by titrating a solution of the unknown with a standard solution of dichromate, cr2o72−. the net ionic equation is 6fe2+(aq)+cr2o72−(aq)+14h+(aq)→6fe3 +(aq)+2cr3+(aq)+7h2o(aq) part a the titration of 25.0 ml of an iron(ii) solution required 18.0 ml of a 0.230 m solution of dichromate to reach the equivalence point. what is the molarity of the iron(ii) solution?
Answers: 1

Chemistry, 23.06.2019 16:00, Bladedrose2351
Express your answer using two significant figures. 1.7 km^2
Answers: 1

Chemistry, 23.06.2019 17:30, lyne29
Which of the following elements would you expect to have the highest ionization energy value, and why? a. chlorine (cl), because it has a low effective nuclear charge and large radius b. fluorine (f), because it has a large radius and naturally forms a negative ion c. lithium (li), because it has a small radius and naturally forms a positive ion d. neon (ne), because it has a high effective nuclear charge and small radius
Answers: 2

Chemistry, 23.06.2019 18:30, hannahbannana98
The electron–pair geometry of a molecule is tetrahedral. what is its bond angle if there are no lone pairs of electrons?
Answers: 2
Do you know the correct answer?
In an experiment, hydrochloric acid reacted with different volumes of sodium thiosulfate in water. A...
Questions in other subjects:







Mathematics, 07.12.2020 23:40

Chemistry, 07.12.2020 23:40







