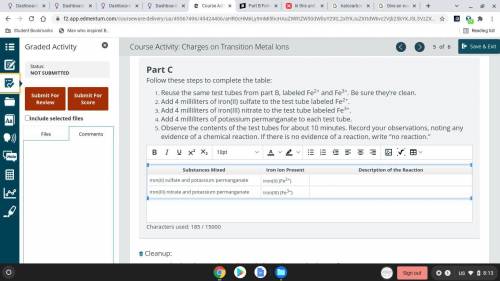
Part C
Follow these steps to complete the table:
Reuse the same test tubes from part B...

Chemistry, 03.02.2022 06:10, nforrestall
Part C
Follow these steps to complete the table:
Reuse the same test tubes from part B, labeled Fe2+ and Fe3+. Be sure they’re clean.
Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe2+.
Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe3+.
Add 4 milliliters of potassium permanganate to each test tube.
Observe the contents of the test tubes for about 10 minutes. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a reaction, write “no reaction.”

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:30, lizethdominguez037
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 05:50, mrylenastewart
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 10:40, trinityanne1738
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 12:30, skaterwolf1317
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Physics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01



Chemistry, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01

Mathematics, 13.10.2020 02:01


Mathematics, 13.10.2020 02:01

Computers and Technology, 13.10.2020 02:01






