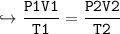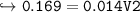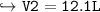Chemistry, 01.02.2022 20:10, markusovaevelyn532
15.0 L of an ideal gas at 298 K and 3.36 atm are heated to 383 K with a new pressure of 5.40 atm. What is the new volume in liters?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, thechocolatblanc
During which movies do spring tides new moon first quarter waxing gibbous waxing
Answers: 1

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 08:30, ebigham5117
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
Do you know the correct answer?
15.0 L of an ideal gas at 298 K and 3.36 atm are heated to 383 K with a new pressure of 5.40 atm. Wh...
Questions in other subjects:




History, 12.01.2021 19:30

Mathematics, 12.01.2021 19:30

Social Studies, 12.01.2021 19:30


Physics, 12.01.2021 19:30

Mathematics, 12.01.2021 19:30