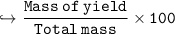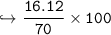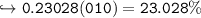Chemistry, 01.02.2022 18:00, lottie2306
In this reaction: Mg (s) + I₂ (s) → MgI₂ (s), if 10.0 g of Mg reacts with 60.0 g of I₂, and 53.88 g of MgI₂ form, what is the percent yield?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:00, slugmilk1090
The agent of mechanical weathering in which rock is worn away by the grinding action of other rock particles is call
Answers: 1

Chemistry, 22.06.2019 19:00, innocentman69
How does a catalyst increase the speed of a reaction? a. the catalyst eliminates the activated complex stage, allowing products to form immediately. b. the catalyst lowers the energy level of the reactants, making it easier for them to react. c. the catalyst makes it easier for the activated complex to form, lowering the activation energy. d. the catalyst raises the energy level of the products, making the reaction finish sooner. reset next
Answers: 1
Do you know the correct answer?
In this reaction: Mg (s) + I₂ (s) → MgI₂ (s), if 10.0 g of Mg reacts with 60.0 g of I₂, and 53.88 g...
Questions in other subjects:

Physics, 09.01.2020 10:31









Mathematics, 09.01.2020 10:31