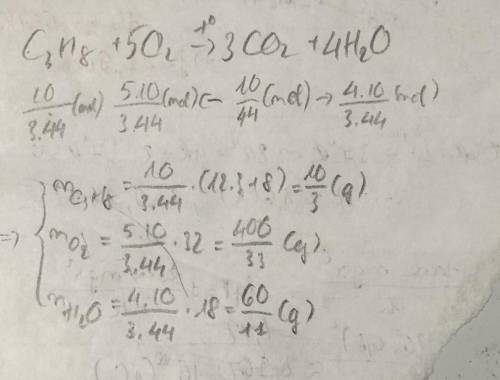Chemistry, 31.01.2022 14:00, Rogeartest4
During combustion reaction of propane (C3H8) the amount of CO2 gas was 10 grams. Calculate the mass of burnt propane, oxygen and water from the reaction? Write and balance the equation. Pls write with explanation)))

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, sbhishop19
Balance this equation co2(g) + h2o (g) show that the balanced equation obeys the law if conversation of mass
Answers: 1

Chemistry, 22.06.2019 00:30, natalie1755
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3


Chemistry, 22.06.2019 13:30, xojade
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1
Do you know the correct answer?
During combustion reaction of propane (C3H8) the amount of CO2 gas was 10 grams. Calculate the mass...
Questions in other subjects:

Mathematics, 03.12.2021 18:40



History, 03.12.2021 18:40



Mathematics, 03.12.2021 18:40

Arts, 03.12.2021 18:40

Mathematics, 03.12.2021 18:40