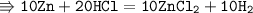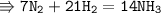Chemistry, 29.01.2022 23:30, annaclaire22
For each chemical equation below, write the number of product molecules that will form from the reaction. Then, circle the limiting reactant. (Note: The coefficients in front of the reactants indicate the number of reactant molecules or atoms present.)
10Zn + 16HCl = ZnCl2 + H2
7N2 + 9H2 = NH3

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, carlybeavers50
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2


Chemistry, 22.06.2019 05:20, jtingley0502
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 05:30, ayoismeisjjjjuan
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1
Do you know the correct answer?
For each chemical equation below, write the number of product molecules that will form from the reac...
Questions in other subjects:

History, 21.01.2021 17:40



Mathematics, 21.01.2021 17:40

English, 21.01.2021 17:40




Mathematics, 21.01.2021 17:40

Mathematics, 21.01.2021 17:40