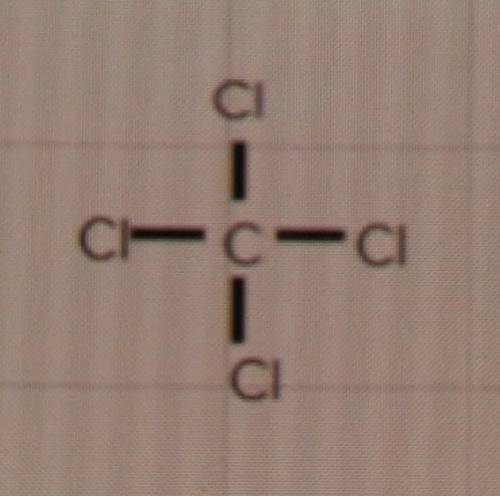Chemistry, 23.01.2022 20:50, Ilcienne6590
Hi! Can someone help me with this???
The question: Will these compounds form single, double or triple bonds?
d) CCℓ4
I'm just a bit confused because the Lewis structures look like it would be a 4 bond, but I don't know if thats even a thing. My only options are single, double or triple.
Thank you!

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:30, palcochran1313
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 08:30, kkelley9223
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 08:40, jaueuxsn
Ageologist determines that a sample of a mineral can't be scratched by a steel nail but can be scratched by a masonry drill bit. based on this information, the sample mineral has to be softer than a. orthoclase. b. fluorite. c. apatite. d. corundum.
Answers: 2

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1
Do you know the correct answer?
Hi! Can someone help me with this???
The question: Will these compounds form single, double or tri...
Questions in other subjects:

Mathematics, 09.05.2021 18:10


Mathematics, 09.05.2021 18:10


Mathematics, 09.05.2021 18:20


World Languages, 09.05.2021 18:20

Mathematics, 09.05.2021 18:20

Biology, 09.05.2021 18:20

Mathematics, 09.05.2021 18:20