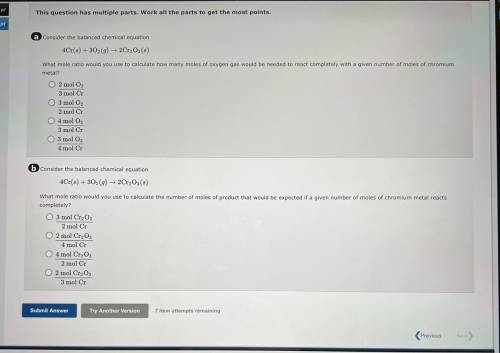
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would yo...

Chemistry, 20.01.2022 19:30, lLavenderl
A consider the balanced chemical equation
4Cr(6) + 30(9) 2Cr, Os(8)
What mole ratio would you use to calculate how many moles of oxygen gas would be needed to react completely with a given number of moles of chromium
metal?
O 2 mol O,
3 mol Cr
O 3 mol O2
2 mol Cr
04 mol O
3 mol C
O 3 mol O2
4 mol Cr
6 Consider the balanced chemical equation
4Cr(6) +302 (9) ► 2Cr, Os()
What mole ratio would you use to calculate the number of moles of product that would be expected if a given number of moles of chromium metal reacts
completely?
O 3 mol Cr2O3
2 mol C
O 2 mol CrgO;
4 mol Cr
O 4 mol Cr2O3
2 mol Cr
O 2 mol Cryo,
3 mol Cr

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, jrfranckowiak
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 18:00, faithabossard
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 22.06.2019 23:00, hailey5campbelp7d1c0
In which region is the substance in both the solid phase and the liquid phase? 1 2. 3 4 mark this and return save and exit next
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 13.11.2020 18:50

History, 13.11.2020 18:50

Mathematics, 13.11.2020 18:50

English, 13.11.2020 18:50

Mathematics, 13.11.2020 18:50

Social Studies, 13.11.2020 18:50




Mathematics, 13.11.2020 18:50






