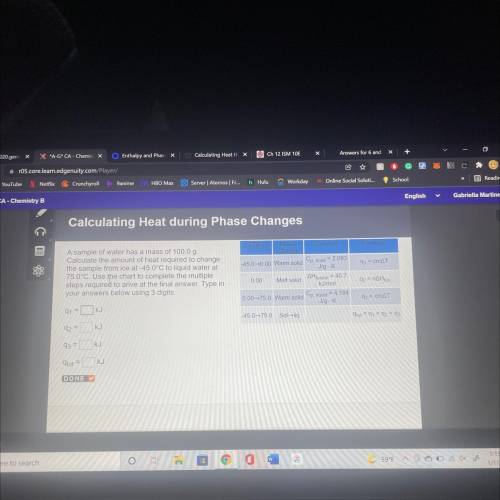
A sample of water has a mass of 100.00
Calculate the amount of heat required to change
the s...

Chemistry, 19.01.2022 14:00, somethingar183
A sample of water has a mass of 100.00
Calculate the amount of heat required to change
the sample from ice at 450°C to liquid water at
78.0°C. Use the chart to complete the multiple
steps required to arrive at the final answer. Type in your answers below using 3 digits.
Q1= KJ
Q2= KJ
Q3= KJ
q(tot)= KJ

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, hemolelekeakua
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 05:00, adjjones2011
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1

Chemistry, 22.06.2019 14:30, Priskittles
What state of matter is ice a. liquid b. element c. solid d. gas
Answers: 1

Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3
Do you know the correct answer?
Questions in other subjects:


English, 26.10.2021 21:00


Biology, 26.10.2021 21:10

Business, 26.10.2021 21:10

Biology, 26.10.2021 21:10

Biology, 26.10.2021 21:10


History, 26.10.2021 21:10

Mathematics, 26.10.2021 21:10






