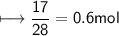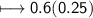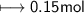Chemistry, 16.01.2022 05:50, ashleyvalles16
Calculate the number of moles of SO2 required to produce 17 g of CO in the following chemical equation: 5 C + 2 SO2 → CS2 + 4 CO

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, maddietomlinson113
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a. inner transition b. noble gases c. representative d. transition
Answers: 2

Chemistry, 22.06.2019 02:00, rosie20052019
Which of the following happens during cell division? (a) energy is created (b) waste is eliminated (c) carbon dioxide is released (d) damaged cells are replaced
Answers: 1

Chemistry, 22.06.2019 04:40, deedee363
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3
Do you know the correct answer?
Calculate the number of moles of SO2 required to produce 17 g of CO in the following chemical equati...
Questions in other subjects:



Social Studies, 04.03.2021 03:00

Chemistry, 04.03.2021 03:00

Mathematics, 04.03.2021 03:00

Mathematics, 04.03.2021 03:00


Mathematics, 04.03.2021 03:00

English, 04.03.2021 03:00

Mathematics, 04.03.2021 03:00