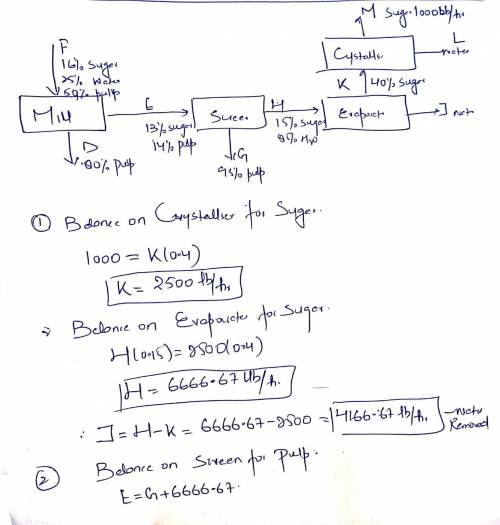
Cane sugar is being manufactured at the rate of 500 kg/hr from sugar canes that have the following composition (% by weight):
Sugar = 16%
Water = 25 %
Pulp = 59 %
The canes are crushed in a mill to mechanically extract the juice and the cellulosic residue (called bagasse) is discarded. The juice containing 13 percent sugar and 14 percent pulp is filtered on vibrating screens to remove the pulp. The resulting clear syrup containing 15 % sugar (rest water) is first concentrated to 40 % in an evaporator and then fed to an evaporator-crystalliser where further water is removed and sugar crystals separate out. Bagasse discarded from the mill contains 80% pulp and solids retained on the screens 95% pulp. Calculate:
a) Mass of canes required per hour
b) Mass of water removed in evaporator per hour
c) Composition (% by weight) of solids retained on screens
d) Percentage of sugar lost in bagasse

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 22.06.2019 22:20, icantspeakengles
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2

Chemistry, 23.06.2019 14:50, aleilyg2005
Use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 use the bond energies provided to estimate δh°rxn for the reaction below. ch3oh(l) + 2 o2(g) → co2(g) + 2 h2o(g) δh°rxn = ? bond bond energy (kj/mol) c-h 414 c-o 360 c=o 799 o=o 498 o-h 464 +473 kj +206 kj -392 kj -91 kj -486 kj
Answers: 1
Do you know the correct answer?
Cane sugar is being manufactured at the rate of 500 kg/hr from sugar canes that have the following c...
Questions in other subjects: