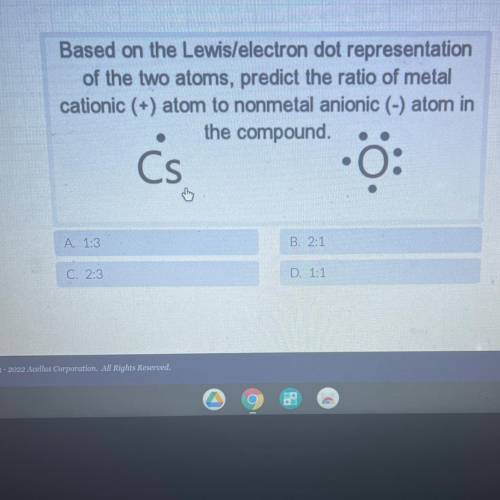
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
...

Chemistry, 12.01.2022 01:00, jesussaves333
Based on the Lewis/electron dot representation
of the two atoms, predict the ratio of metal
cationic (+) atom to nonmetal anionic (-) atom in
the compound.
Cs
0:
A. 1:3
B. 2:1
C. 2:3
D. 1:1

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:30, thatonestudent2271
If 3.00 g of titanium metal is reacted with 6.00 g of chlorine gas, cl2, to form 7.7 g of titanium (iv) chloride in a combination reaction, what is the percent yield of the product?
Answers: 1

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 04.12.2021 01:10







Social Studies, 04.12.2021 01:10







