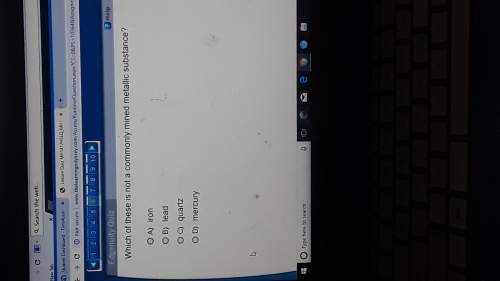
Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:40, sadcase85
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na, so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

Chemistry, 22.06.2019 09:00, SilverTheAmarok
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 2


Chemistry, 23.06.2019 04:00, hailey200127
What is the volume of 2.5 moles of nitrogen gas (n2)at standard temperature and pressure (stp)?
Answers: 1
Do you know the correct answer?
Calculate the energy required to produce 183 grams Cl2O7 on the basis of the following balanced equa...
Questions in other subjects:




Biology, 14.11.2019 18:31

History, 14.11.2019 18:31

History, 14.11.2019 18:31


Social Studies, 14.11.2019 18:31

Geography, 14.11.2019 18:31

Mathematics, 14.11.2019 18:31