Which example has 1.2×10^24 hydrogen atoms?
2 moles of ammonia (NH3)
2 moles of hydroge...

Chemistry, 05.01.2022 20:50, lineaeriksen
Which example has 1.2×10^24 hydrogen atoms?
2 moles of ammonia (NH3)
2 moles of hydrogen gas (H2)
1 mole of water (H2O)
1 mole of methane (CH4)
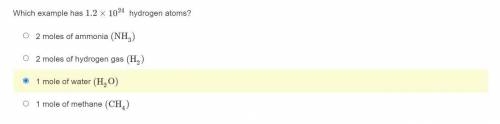

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 08:30, ayaanwaseem
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
Do you know the correct answer?
Questions in other subjects:








Mathematics, 06.07.2019 20:10







