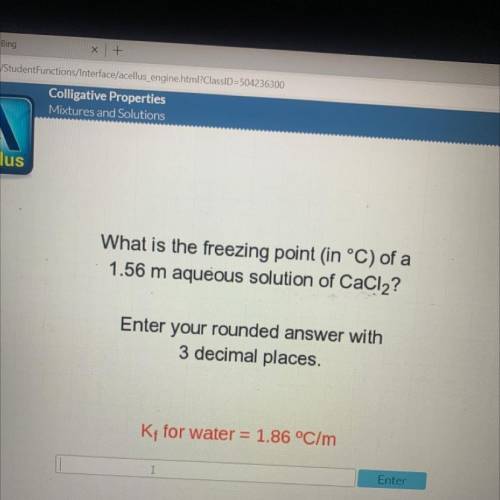
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded...

Chemistry, 02.01.2022 14:00, edimilperdomo
What is the freezing point in °C) of a
1.56 m aqueous solution of CaCl2?
Enter your rounded answer with
3 decimal places.
Kt for water = 1.86 °C/m

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 22:00, jlegrand9098
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2
Do you know the correct answer?
Questions in other subjects:

Mathematics, 01.06.2021 23:20


History, 01.06.2021 23:20

Business, 01.06.2021 23:20

Chemistry, 01.06.2021 23:20



Mathematics, 01.06.2021 23:20


Mathematics, 01.06.2021 23:20






