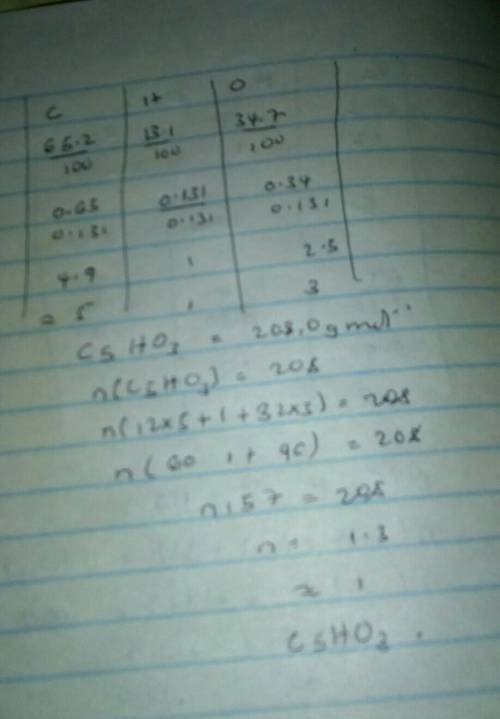Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:00, tbiles99
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1


Chemistry, 22.06.2019 20:30, kittybatch345
Is a chemical message sent by another individual.
Answers: 1
Do you know the correct answer?
#6: A compound contains 65.2% carbon, 13.1% hydrogen and 34.7% oxygen. If its molar mass is 208.0 g/...
Questions in other subjects:

Mathematics, 28.08.2019 06:10

History, 28.08.2019 06:10








Mathematics, 28.08.2019 06:10