Given the equation
N2(g) +3H2 (g)--> 2NH3 (g)
1 mole of N2 gas is needed to completely re...

Chemistry, 25.12.2021 17:40, levicorey846
Given the equation
N2(g) +3H2 (g)--> 2NH3 (g)
1 mole of N2 gas is needed to completely react with 3 moles of H2 gas.
How many molecules of H2 gas are needed?


Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:30, homeschool0123
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:00, halohero7
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2

Do you know the correct answer?
Questions in other subjects:



Computers and Technology, 07.04.2021 02:30

Biology, 07.04.2021 02:30

Mathematics, 07.04.2021 02:30

Mathematics, 07.04.2021 02:30

Mathematics, 07.04.2021 02:30

Mathematics, 07.04.2021 02:30

Advanced Placement (AP), 07.04.2021 02:30


 molecules H2
molecules H2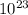 molecules= 1.81 x
molecules= 1.81 x 



