
Chemistry, 22.12.2021 03:10, haileyhale5
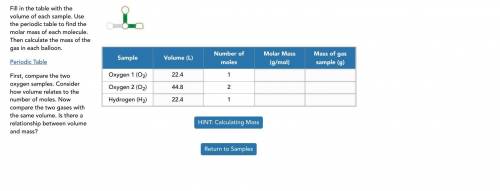
Fill in the table with the volume of each sample. Use the periodic table to find the molar mass of each molecule. Then calculate the mass of the gas in each balloon.
Periodic Table
First, compare the two oxygen samples. Consider how volume relates to the number of moles. Now compare the two gases with the same volume. Is there a relationship between volume and mass?
2. How can you determine the volume of a gas from the number of moles of a gas (at STP)? Use the pattern you identified in the data table to find the mass of an 89.6 L sample of helium. Helium has a molar mass of 4.0 g/mol.


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, dyanaycooper13
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 22.06.2019 16:00, jrocklove7825
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 16:10, sierram298
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1
Do you know the correct answer?
Fill in the table with the volume of each sample. Use the periodic table to find the molar mass of e...
Questions in other subjects:



Social Studies, 02.09.2021 01:00


English, 02.09.2021 01:00


Mathematics, 02.09.2021 01:00


History, 02.09.2021 01:00






