
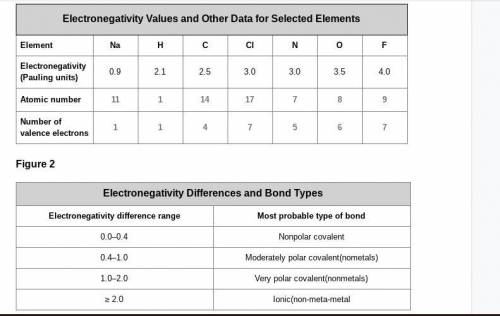
A cesium (Cs) atom has an atomic number of 55 and one electron in its outer shell. Considering the data in Figure 1 and Figure 2, what electronegativity value would you expect it to have, and what kind of bond is it likely to form with a chlorine atom? Explain your reasoning.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, KeiraDawn
An engineering team designs a new rocket that is faster and lighter than any other model being produced. however, the materials end up being so expensive that no company can afford to buy them. which step of the engineering process should have addressed this problem? a. know the background. b. evaluate the results. c. identify a need. d. do the work.
Answers: 2

Chemistry, 22.06.2019 07:10, nasrul3725
Remember to use the proper number of significant figures and leading zeros in all calculations. gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 09:40, loveoneonly9153
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
Do you know the correct answer?
A cesium (Cs) atom has an atomic number of 55 and one electron in its outer shell. Considering the d...
Questions in other subjects:

History, 03.10.2020 01:01




Mathematics, 03.10.2020 01:01





History, 03.10.2020 01:01






