
Chemistry, 16.12.2021 01:20, breanastone15
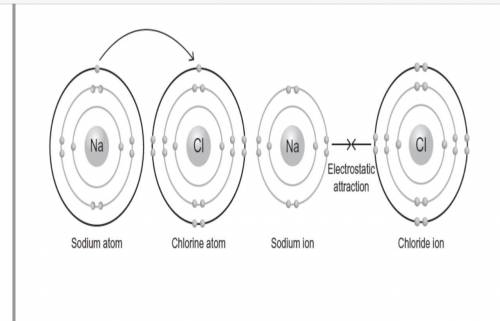
The following figure shows the bond formation of sodium chloride, which is the compound commonly known as table salt.
Which of the following statements are true once the sodium chloride bond formation shown in the figure takes place? Select all that apply.
A. The resulting sodium cation has a stable octet in its valence shell.
B. The resulting chlorine anion has a stable octet in its valence shell.
C. The electrostatic attraction between the positive sodium ion and negative chlorine ion forms a nonpolar bond.
D. The electrostatic attraction between the positive sodium ion and negative chlorine ion forms a polar covalent bond.
E. The electrostatic attraction between the positive sodium ion and negative chlorine ion forms an ionic bond.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:00, alaina3792
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 06:00, applejulianamoreno
If a polyatomic ionic compound has gained two hydrogen ions, then how does its name begin?
Answers: 3

Chemistry, 22.06.2019 09:20, kevinhernandez582
What will most likely happen when two bromine atoms bond together?
Answers: 3
Do you know the correct answer?
The following figure shows the bond formation of sodium chloride, which is the compound commonly kno...
Questions in other subjects:


Advanced Placement (AP), 03.01.2020 19:31

English, 03.01.2020 19:31




English, 03.01.2020 19:31

Mathematics, 03.01.2020 19:31








