
Chemistry, 15.12.2021 23:20, juelchasse
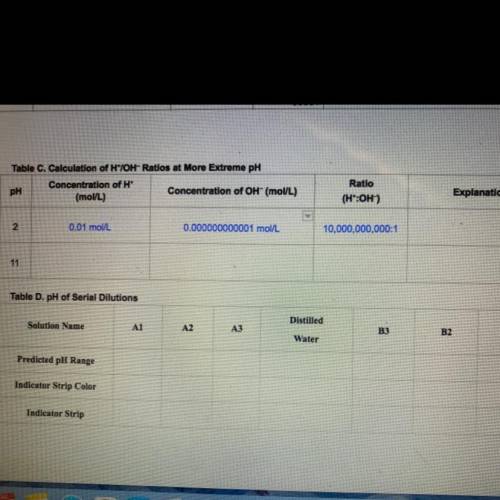
In Step 5, you will calculate H*/OH- ratios for more
extreme pH solutions. Find the concentration of H* ions
to OH- ions listed in Table B of your Student Guide for a
solution at a pH = 2. Then divide the H* concentration by
the OH concentration. Record these concentrations and
ratio in Table C.
I know the answer I just don’t know what to put for the explanation on the table.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, lylessd423
Two atoms interact with each other as shown by the equation. complete the equation by filling in the missing parts. 1 2 3 4 5 h he li
Answers: 2

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 23.06.2019 02:10, sativataurus
Detrimental the length of the object shown 1. 97.8mm 2. 97.80 mm 3. 97mm 4. 98mm
Answers: 2
Do you know the correct answer?
In Step 5, you will calculate H*/OH- ratios for more
extreme pH solutions. Find the concentration...
Questions in other subjects:

Physics, 15.09.2019 20:10

Mathematics, 15.09.2019 20:10

Spanish, 15.09.2019 20:10

History, 15.09.2019 20:10



Mathematics, 15.09.2019 20:10


Mathematics, 15.09.2019 20:10

Mathematics, 15.09.2019 20:10






