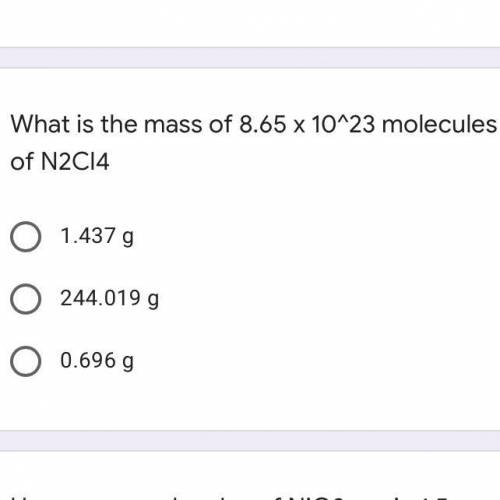
What is the mass of 8.65 x 10^23 molecules of N2Cl4
A. 1.437 g
B. 244.019 g
C. 0.696 g...

Chemistry, 14.12.2021 21:50, baeethtsadia
What is the mass of 8.65 x 10^23 molecules of N2Cl4
A. 1.437 g
B. 244.019 g
C. 0.696 g

Answers: 3
Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Biology, 29.01.2020 07:56

Arts, 29.01.2020 07:56


History, 29.01.2020 07:56


Computers and Technology, 29.01.2020 07:56




Social Studies, 29.01.2020 07:56






