
Chemistry, 14.12.2021 21:30, slawson4328
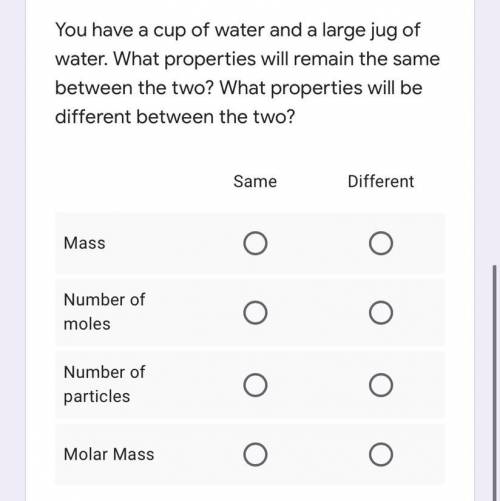
You have a cup of water and a large jug of water. What properties will remain the same between the two? What properties will be different between the two?
Mass: Same or different
Number of moles: Same or different
Number of particles: Same or different
Molar mass: Same or different

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, kitt90335
Asample contains 16.75 g of the radioisotope u-236 and 50.25 g of its daughter isotope, th-232. how long did it take for decay to take place if one half-life of u-236 is 23 million years? 46 million years 69 million years 92 million years 115 million years
Answers: 3

Chemistry, 21.06.2019 23:00, DarcieMATHlin2589
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 23.06.2019 03:50, KAITLYN007
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer.
Answers: 1

Chemistry, 23.06.2019 06:00, lanaiheart7
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
Do you know the correct answer?
You have a cup of water and a large jug of water. What properties will remain the same between the t...
Questions in other subjects:

Biology, 09.04.2021 01:10


Mathematics, 09.04.2021 01:10

English, 09.04.2021 01:10


History, 09.04.2021 01:10

History, 09.04.2021 01:10

Social Studies, 09.04.2021 01:10


Mathematics, 09.04.2021 01:10






