
Chemistry, 13.12.2021 18:50, kimlyn58p0wyn0
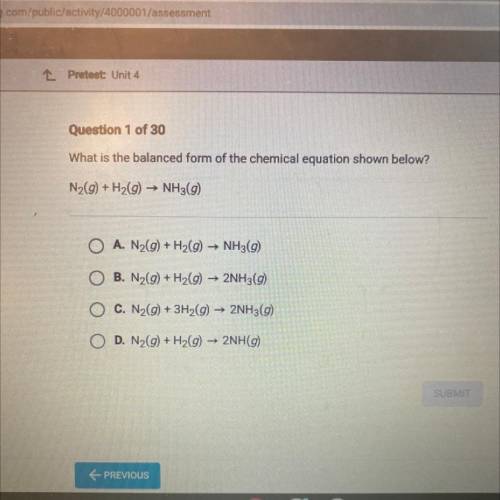
What is the balanced form of the chemical equation shown below?
N2(g) + H2(9) ► NH3(9)
O A. N2(9) + H2(9) ► NH3(9)
B. N2(9) + H2(g) → 2NH3(9)
)
O C. N2(g) + 3H2(g) → 2NH3(9)
O 9
D. N2(9) + H2(g) → 2NH(9)

Answers: 3
Other questions on the subject: Chemistry




Chemistry, 22.06.2019 21:30, steven0448
An atomic nucleus is composed ofa)protons. b)protons and neutrons. c)protons and electrons. d)protons, neutrons, and electrons.
Answers: 1
Do you know the correct answer?
What is the balanced form of the chemical equation shown below?
N2(g) + H2(9) ► NH3(9)
O A....
O A....
Questions in other subjects:


Geography, 04.12.2019 08:31


History, 04.12.2019 08:31

Mathematics, 04.12.2019 08:31

Social Studies, 04.12.2019 08:31

Social Studies, 04.12.2019 08:31

Health, 04.12.2019 08:31

History, 04.12.2019 08:31

Mathematics, 04.12.2019 08:31







