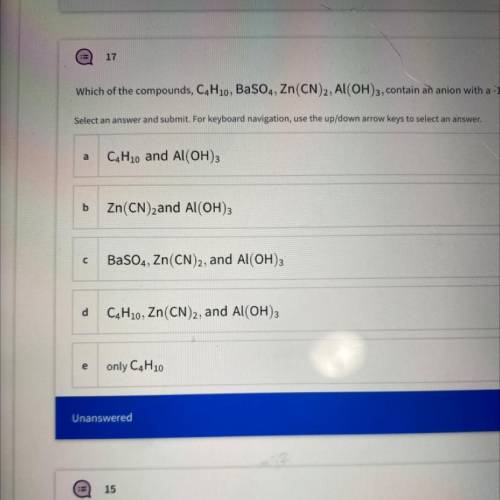
Which of the compounds, C4H10, BaSO4, Zn(CN)2, Al(OH)3, contain an anion with a -1 charge?
...

Chemistry, 13.12.2021 07:30, notearslefttocry14
Which of the compounds, C4H10, BaSO4, Zn(CN)2, Al(OH)3, contain an anion with a -1 charge?

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 21.06.2019 21:00, alexmarche4675
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Do you know the correct answer?
Questions in other subjects:



Mathematics, 13.01.2020 19:31

Computers and Technology, 13.01.2020 19:31

Computers and Technology, 13.01.2020 19:31


Health, 13.01.2020 19:31


Mathematics, 13.01.2020 20:31

Mathematics, 13.01.2020 20:31






